Otsuka Pharmaceutical Co., Ltd.
Otsuka Submits a Japan NDA for Nalmefene, a Drug to Reduce Alcohol Consumption in Alcohol-Dependent Patients
- Nalmefene suppresses the urge in people with alcohol dependency to drink alcohol when it is taken before or at the time the urge is felt
- The primary endpoint of a reduction in heavy-drinking days and the secondary endpoint of a reduction in total alcohol consumption were met in the trial.
- Nalmefene, if approved, would provide a new option to patients struggling with alcohol dependency
Otsuka and Lundbeck today announce the submission by Otsuka of a new drug application (NDA) for nalmefene hydrochloride (nalmefene) for patients with alcohol dependency.
Among mental disorders, alcohol dependence not only impairs one's health but also has significant social and economic impacts, reinforcing the urgency to find new ways to help address the issue. Nalmefene, co-developed in Japan by Otsuka and Lundbeck, is the first treatment that can be taken when a concern about, or the urge itself arises to consume alcohol. It is an antagonist that binds to and blocks certain opioid receptors in the brain. This drug candidate is anticipated as a potentially continuous treatment option for the purpose of reducing alcohol consumption and helping patients aim at social reintegration.
The NDA was supported by data from a comparative trial of patients with an alcohol dependency who were randomized to treatment with nalmefene or placebo in conjunction with psychosocial intervention focused on treatment adherence. Nalmefene was administered in fixed doses of either 10 mg per day or 20 mg per day. During the 24-week trial period, the effectiveness and safety of nalmefene was assessed.
The primary trial endpoint was the change in the number of heavy-drinking days*1 from baseline. A key secondary endpoint was the change in total alcohol consumption*2 from baseline. An important objective was to observe the extent to which consumption of alcohol was moderated in individuals with alcohol dependence.
- 1Heavy-drinking days (HDDs) : number of days per month with alcohol consumption greater than 60 grams per day for males and greater than 40 grams per day for females
- 2Total alcohol consumption (TAC): Mean average daily alcohol consumption over a one-month period (grams per day)
In the trial, a statistically significant difference (P <0.0001) was observed between nalmefene and placebo for the primary endpoint, the number of HDDs from baseline to week 12. A difference was observed for both the 10 mg per day and the 20 mg per day nalmefene groups compared to the placebo group. The effect was maintained through the treatment course.
Furthermore, a statistically significant (P <0.0001) decrease was also observed for both nalmefene groups on the key secondary endpoint, TAC.
Side effects from nalmefene included nausea, dizziness and somnolence and these were mild or moderate in intensity. Dependency and withdrawal symptoms from nalmefene were not observed.
About nalmefene
Nalmefene is a selective opioid receptor ligand. Opioid receptors are widely distributed in the central nervous system and govern the intracerebral reward system, emotional control, pain control. Three subtypes (μ or mu; κ or kappa; δ or delta) are known.
Nalmefene is believed to act as an antagonist on μ and δ receptors and to act as a partial agonist on κ receptors, reducing the urge to consume alcohol.
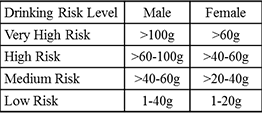
The treatment indication in the EU is to help reduce alcohol consumption in adults with alcohol dependence who consume more than 60 g of alcohol per day (for men) or more than 40 g per day (for women), consumption which places the health of these adults at high risk or very high risk as reflected in the table below.