Otsuka Pharmaceutical Co., Ltd.
Pharmaceuticals
June 8, 2017
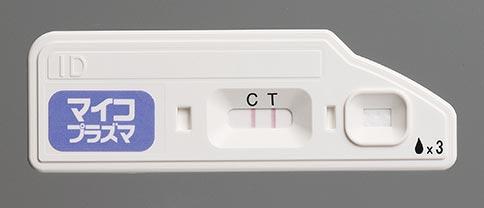
Quick NaviTM-Mycoplasma Diagnostic Kit to Launch in Japan
- Quick NaviTM-Mycoplasma Diagnostic Kit to be launched as a new product of the Quick Navi Series
- As with other products of the series, this simple-to-use diagnostic kit is rapid and displays the result clearly
- Has excellent sensitivity and specificity to mycoplasma antigen.
Otsuka Pharmaceutical Co., Ltd. will commence sales in Japan from July 12, 2017, of the diagnostic test kit Quick NaviTM-Mycoplasma. The kit, which can detect mycoplasma antigens quickly and with high sensitivity, is a new product in the Quick Navi Series of in vitro diagnostics.