Otsuka Pharmaceutical Co., Ltd.
SAMSCA™ Tablets 15mg (tolvaptan), Vasopressin V2-Receptor Antagonist, Receive Regulatory Approval in Japan
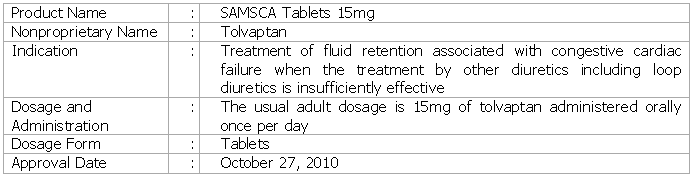
Tokyo, Japan, October 27, 2010 - Otsuka Pharmaceutical Co., Ltd. today announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved SAMSCA™ (nonproprietary name: tolvaptan), a vasopressin V2-receptor antagonist, for the treatment of excess water retention in patients with cardiac failure when the treatment by other diuretics including loop diuretics is ineffective.
SAMSCA is a nonpeptide vasopressin V2-receptor antagonist discovered by Otsuka Pharmaceutical with a unique mechanism of action that selectively blocks the binding of vasopressin to the V2-receptors in the collecting duct of the kidney. The binding of vasopressin with V2-receptors can cause water retention. By inhibiting the effects of vasopressin at the V2-receptor, SAMSCA increases the excretion of free water and reduces the re-absorption of water from urine into the blood without directly affecting the excretion of sodium and other electrolytes.
"Our research and development on vasopressin V2-receptor antagonists, that began in the early 1980s, was in response to requests from the medical profession for a diuretic that excretes only water," said Taro Iwamoto, Ph.D., President and Representative Director, Otsuka Pharmaceutical. "Treatment methods that employed diuretics had remained unchanged for about three decades, and there was a need to improve conventional therapies. Receiving Japanese approval for SAMSCA Tablets is an accomplishment that resulted from our company's extended research and development efforts. We are delighted that we will soon be able to deliver this product to patients and physicians in Japan. It is our hope that SAMSCA Tablets will improve the quality of health in patients where preexisting treatments were not fully effective."
Otsuka Pharmaceutical, since the discovery of SAMSCA, has conducted global development of the product in North America, Europe, Japan and other parts of Asia. SAMSCA was first launched in the United States in June 2009. SAMSCA was launched in European countries including the UK and Germany following approval by the European Commission in August 2009.
Based on its corporate philosophy of "Otsuka-people creating new products for better health worldwide," Otsuka Pharmaceutical Co., Ltd. is dedicated to contributing to the health of people around the world.
About the Phase III clinical trial in Japan
In a Phase III placebo control double-blind comparative trial in Japan to evaluate the efficacy of SAMSCA (Qualification of Efficacy and Safety in the Study of Tolvaptan in Cardiac Edema or QUEST), patients with congestive cardiac failure who were demonstrated to have fluid retention even after the administration of conventional diuretics received either a 15mg oral dosage of SAMSCA or a placebo once a day for seven days. With regard to the primary outcome of change in body weight at the time of final administration, patients in the SAMSCA group were shown to have a significant decline when compared with those in the placebo group. Additionally, improvement was achieved in observations of cardiac edema (carotid dilation, hepatomegaly, edema of the lower extremities) at the time of final administration.
About Fluid Retention in Congestive Cardiac Failure
Edema (fluid retention) resulting from the deterioration in heart pumping function can develop in individuals with congestive cardiac failure. Edema stemming from cardiac heart failure negatively affects patient quality of life including labored breathing and a decline in activity. While diuretics are generally used for the treatment of edema, there have been concerns about the insufficient effectiveness of conventional diuretics for some patients, and about serum electrolyte abnormalities and diminished renal function when diuretics are used in combination or in increased dosages. Additionally, it has been pointed out that restricting fluid intake to manage the body fluid volume in patients can inflict suffering.
Approved information about SAMSCA Tablets