Otsuka Pharmaceutical Co., Ltd.
Antipsychotic Agent ABILIFY® Receives Regulatory Approvals for Additional Indication "Improvement of Manic Symptoms Associated with Bipolar Disorder" and for New Dosage Form "ABILIFY® OD Tablets"
- Early improvement in manic symptoms associated with bipolar disorder*1 shown in Phase III clinical trials
- Long-term continuous use of the drug is possible with less likelihood of drowsiness and other side effects caused by conventional antipsychotic agents
- Simultaneous approval received for ABILIFY OD*2 Tablets, a dosage form that instantly dissolves orally and can be taken without water
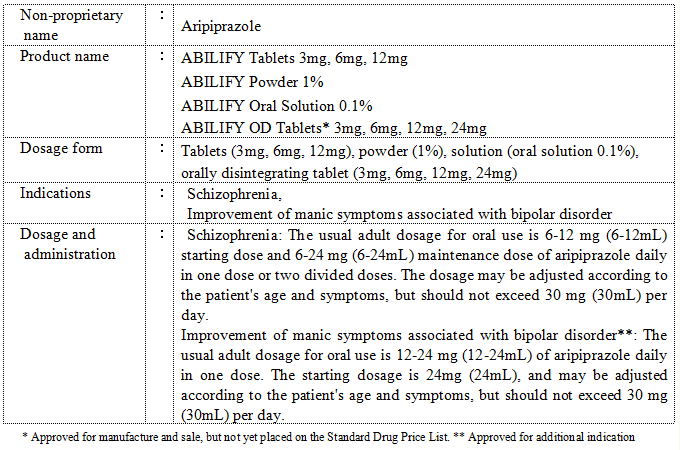
Tokyo, Japan, January 18, 2012 -- Otsuka Pharmaceutical Co., Ltd. (Head Office: Chiyoda-ku, Tokyo, Japan; President: Taro Iwamoto) has received new regulatory approvals in Japan for the antipsychotic agent ABILIFY® (aripiprazole). Specifically, the new approvals are for the additional indication "improvement of manic symptoms associated with bipolar disorder" (in addition to the existing indication of schizophrenia), and for the new dosage form "ABILIFY® OD Tablets".
In Phase III clinical trials conducted as international cooperative trials, ABILIFY was shown to display early improvement of manic symptoms associated with bipolar disorder. A manic state can result in elevated energy levels, with impaired judgment and tendency toward anger that interfere with social ability.While proper adherence to medicine is important in the treatment of bipolar disorder, conventional antipsychotic agents have presented the problem of patients discontinuing use due to side effects such as drowsiness. ABILIFY is less likely to result in these side effects, and is expected to become a first-line drug that can be adhered to without loss of QOL (quality of life).
ABILIFY is the world's first antipsychotic agent that acts as a dopamine D2 receptor partial agonist. Otsuka Pharmaceutical commenced marketing ABILIFY in Japan in June 2006 as a treatment for schizophrenia.
Beginning with regulatory approval in the US in 2004, ABILIFY has received approval in 57 countries and regions in Europe, Asia, and elsewhere for treatment of manic symptoms associated with bipolar disorder.
ABILIFY OD Tablets are a new dosage form that instantly dissolves orally and can be ingested anywhere without water, making the tablets agreeable and easily taken by patients. Otsuka Pharmaceutical intends to market the tablets soon after placement on the Standard Drug Price List.
- *1:Bipolar disorder, which is also referred to as manic- depressive psychosis, is an illness with symptoms that include a repetitive cycle between manic and depressive states.
- *2: An abbreviation of Orally Disintegrating. Unlike regular tablets, the tablets instantly dissolve orally.
About bipolar disorder
Bipolar disorder, which is also referred to as manic- depressive psychosis, is said to have a lifetime prevalence of 0.4%*. Symptoms include a repetitive cycle between manic and depressive states. A manic state is defined by the presence of abnormally elevated energy levels and impaired judgment. In a large number of cases, the individual is unaware of any illness and is unlikely to seek assistance. Signs and symptoms of the depressive phase, on the other hand, include strong feelings of despair. Individuals with this condition will oftentimes seek the support of others. Unable to accept or deal with the reality of the situation, depression can also lead to feelings of hopelessness. In the case of mixed episodes or states, the features of both mania and depression are present at the same time. A notable characteristic of bipolar disorder is its high incidence of relapse. Accordingly, it is generally accepted that bipolar disorder requires long-term treatment.
- *:Research relating to the epidemiological study of mental health: Fiscal 2006 Health and Labour Sciences Research Grant (Mental Health Science)
About ABILIFY
ABILIFY® is described as a dopamine system stabilizer (DSS). It exerts an inhibitory action when large amounts of dopamine are released in the brain and a stimulatory action when only small amounts are released, thus stabilizing dopamine neurons. This mechanism enables the drug to improve both the positive and negative symptoms of schizophrenia that are thought to be induced by dopamine abnormalities. At the same time, the drug can be used for long-term continuous treatment, as it is less likely to cause drowsiness or weight gain. The drug is thought to display the same effect on manic symptoms associated with bipolar disorder as it has on schizophrenia, through the shared medicinal action of inhibiting dopamine neurons. Under various guidelines established worldwide, ABILIFY is recommended as a first-line drug for manic symptoms associated with bipolar disorder, based upon its extensive record of use overseas and on reports from numerous clinical trials.
To date, ABILIFY is sold in 65 countries and regions worldwide including Japan, with global sales of approximately 400 billion yen in fiscal 2010.
Overview of approvals in Japan for ABILIFY