Takeda Pharmaceutical Co., Ltd.
Otsuka Pharmaceutical Co., Ltd.
TAKECAB® Now Available for the Treatment of Acid-related Diseases in Japan
- TAKECAB®, discovered by Takeda for the treatment of acid-related disease, is now available in Japan.
- Takeda and Otsuka executed an agreement at the end of March 2014 to co-promote TAKECAB® (for the treatment of acid-related diseases) in Japan. Under the agreement, the two companies will conduct informational activities for healthcare professionals with the aim of addressing unmet clinical needs in the treatment of acid-related diseases.
- Otsuka will receive from Takeda co-promotion fees based on sales levels of TAKECAB® (in accordance with conditions specified in the agreement).
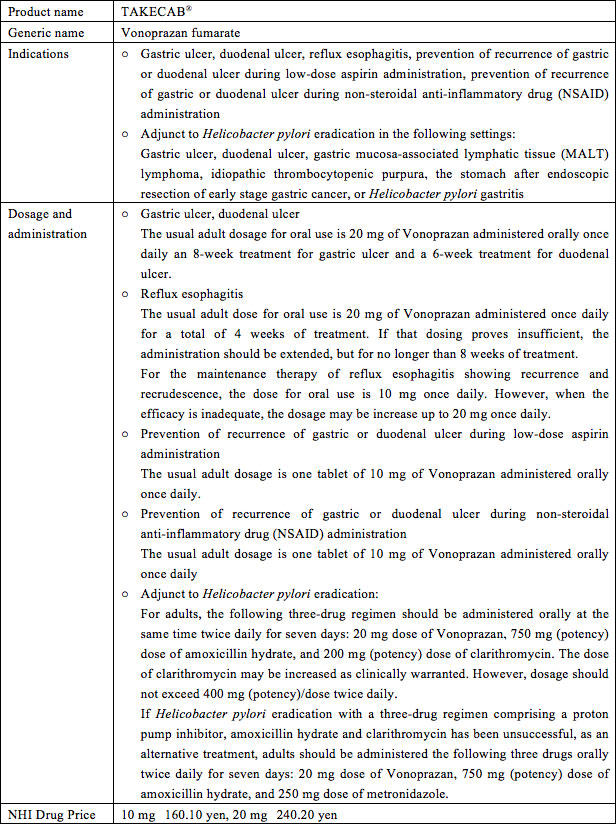
Takeda Pharmaceutical Company Limited (Head office: Chuo-ku, Osaka; President and COO: Christophe Weber; "Takeda") and Otsuka Pharmaceutical Company, Limited (Head office: Chiyoda-ku, Tokyo; President and Representative Director: Tatsuo Higuchi; "Otsuka") announced today that TAKECAB® 10 mg and TAKECAB® 20 mg (generic name: Vonoprazan fumarate, hereafter "TAKECAB"), discovered by Takeda for the treatment of acid-related diseases, is now available in Japan.
TAKECAB, discovered by Takeda, is a new medicine for treating acid-related diseases with a novel mechanism of action called potassium-competitive acid blockers (P-CABs) which competitively inhibits the binding of potassium ions to H+,K+-ATPase (also known as the proton pump) in the final step of gastric acid secretion in gastric parietal cells. Vonoprazan fumarate has fast-acting, strong and sustained acid secretion inhibitory effects. Takeda and Otsuka executed an agreement at the end of March 2014 to co-promote TAKECAB in Japan.
"Ever since our company launched TAKEPRON® more than two decades ago, we have been supporting patients suffering from acid-related diseases and healthcare professionals who are providing them with treatment," said Masato Iwasaki, Ph.D., Director and President, Japan Pharma Business Unit of Takeda. "I am delighted that together with Otsuka, an excellent co-promotion partner, we are able to offer a new treatment option to address the unmet medical needs in the gastrointestinal field. Through TAKECAB, we will make further contributions to the treatment of acid-related disease."
"From diagnosis through to treatment Otsuka is contributing broadly in the gastrointestinal field - Mucosta® is a novel medicine for gastric ulcers and our diagnostic system for helicobacter pylori infections is an excellent complement to it," said Susumu Tamai, Executive Director and Executive Operating Officer, Pharmaceutical Marketing Headquarters of Otsuka. "We are very enthusiastic about entering this collaboration with Takeda in Japan on TAKECAB, an eagerly awaited new treatment for acid-related diseases. We strive to develop innovative medicines and diagnostic drugs to improve the quality of life of many patients."
References
About the co-promotion agreement
The details of this agreement are as below:
- Takeda will receive from Otsuka an up-front payment of 20 billion yen and a milestone payment upon regulatory approval.
- Otsuka will receive from Takeda a co-promotion fee based on the sales amount (based on conditions specified in the contract).
- Territory: Japan
Further details are not disclosed.





