Otsuka Pharmaceutical Co., Ltd.
Takeda Obtains NDA Approval for Helicobacter pylori* Eradication triple-drug Blister Packs Containing TAKECAB tablet, "VONOSAP® pack 400", "VONOSAP® pack 800" and "VONOPION® pack" in Japan
- Takeda has obtained New Drug Application approval in Japan for "VONOSAP® pack 400" and "VONOSAP® pack 800", each being a triple-drug blister pack containing the potassium-competitive acid blocker "TAKECAB® tablet", developed by Takeda, for primary eradication of Helicobacter pylori (H. pylori).
- Approval was also received for "VONOPION® pack", a triple-drug blister pack containing "TAKECAB® tablet" for secondary eradication of H. pylori.
- As per the agreement reached at the end of March 2014 for co-promotion of "TAKECAB® tablet" in Japan, Takeda and Otsuka will implement informational activities on "VONOSAP® pack 400", "VONOSAP® pack 800" and "VONOPION® pack" for eradication of H. pylori, thereby addressing the healthcare needs in acid-related disorders.
- Otsuka will be compensated for all sales generated by its sales force for "TAKECAB® tablet" as well for the TAKECAB®-containing drug packs, "VONOSAP® pack" and "VONOPION® pack".
Takeda Pharmaceutical Company Limited (Head office, Chuo-ku, Osaka; President and COO, Christophe Weber; hereafter "Takeda") and Otsuka Pharmaceutical Co., Ltd. (Head office, Chiyoda-ku, Tokyo; President and Representative Director, Tatsuo Higuchi; hereafter "Otsuka") announced that Takeda has obtained New Drug Application approval from the Ministry of Health, Labour and Welfare for "VONOSAP® pack 400", "VONOSAP® pack 800" (hereafter "VONOSAP") and "VONOPION® pack" (hereafter "VONOPION") for H. pylori eradication.
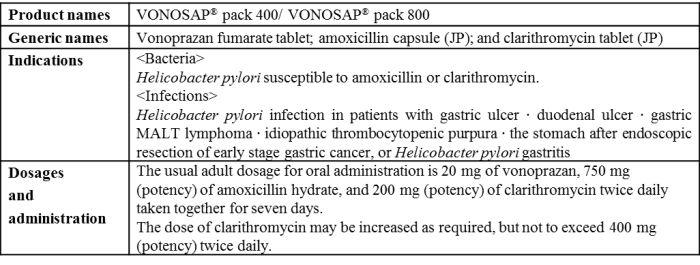
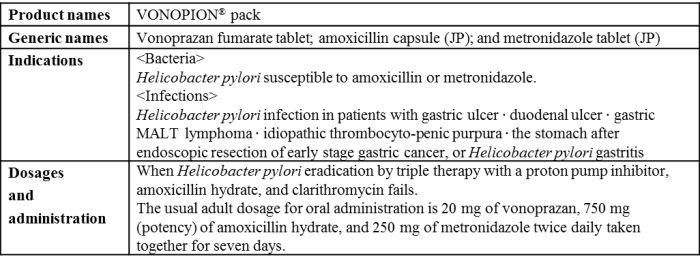
"VONOSAP" is a triple-drug blister pack combining the acid suppressant (also called a potassium-competitive acid blocker (P-CAB) based on its mechanisms of action) "TAKECAB® tablet" (generic name, vonoprazan fumarate; hereafter "TAKECAB") developed by Takeda, "Amolin® capsule" (generic name, amoxicillin; hereafter "Amolin") and "Clarith® tablet" (generic name, clarithromycin) for primary eradication of H. pylori and "VONOPION" is a triple-drug blister pack containing "TAKECAB", "Amolin" and "Fragile® tablet" (generic name, metronidazole) for secondary eradication of H. pylori.
Combining a daily dose of each of the two antibiotics used for H. pylori eradication with TAKECAB, which is known to exert potent and sustained acid-inhibitory effects, these blister packs have been developed to improve patient adherence by ensuring that each component drug is taken without fail. With the added benefit of convenience of use, these blister packs are also expected to provide an alternative treatment option for healthcare professionals to effectively treat their patients. Otsuka and Takeda also hope to further contribute to improving H. pylori eradication therapy in Japan.
- Helicobacter pylori are one type of bacteria present in the human stomach and are primarily known to be implicated in the onset of peptic ulcer. H. pylori eradication therapy has been shown to be effective in preventing peptic ulcer recurrence with markedly low peptic ulcer recurrence rates reported following treatment, thus providing substantial benefits to patients with H. pylori infection.
Overview of VONOSAP
Overview of VONOPION
Details of co-promotion agreement
- Takeda is to receive from Otsuka an up-front payment of 20 billion yen and a milestone payment upon receiving regulatory approval.
- Otsuka is to receive from Takeda a co-promotion fee based on the sales volume (based on conditions specified in the contract).
- Applicable drugs: TAKECAB and the single packs containing TAKECAB
- Territory: Japan
Further details are not disclosed.





