Otsuka Pharmaceutical Co., Ltd.
Nalmefene, a Drug Candidate to Reduce Alcohol Consumption, Shows Positive Results from Phase III Trial in Japan
- Nalmefene suppresses the urge in people with alcohol dependency to drink alcohol when it is taken before or at the time the urge is felt
- The P-III clinical trial achieved its primary and secondary endpoints
Otsuka Pharmaceutical Co., Ltd. (Otsuka) and H. Lundbeck A/S (Lundbeck) announce topline, positive results in Japan from a phase III comparative clinical trial and a follow-on, long-term extension study of nalmefene hydrochloride (nalmefene) in participants with an alcohol dependency. The comparative trial assessed the drug candidate's efficacy and safety relative to placebo, as well as dose responsiveness. The extension study assessed the candidate's long-term efficacy and safety.
The comparative trial was a multicenter, double-blind, three-parallel-group trial comprised of approximately 660 participants with an alcohol dependency who were randomized to treatment with nalmefene or placebo in conjunction with psychosocial intervention focused on treatment adherence and reducing alcohol consumption. Nalmefene was administered in fixed doses of either 10 mg per day or 20 mg per day. During the 24-week trial period, the effectiveness and safety of nalmefene was assessed versus placebo.
The open-label, long-term extension study was based on approximately 400 participants who had completed the 24-week trial and who granted consent to be administered nalmefene in a dose of 20 mg per day for an additional 24 weeks. At the end of the 24 weeks, treatment was terminated and the active-comparator arm and placebo-control arm participants were observed for a four-week period. Comparative effectiveness and safety, as well any dependency on nalmefene, were assessed.
The primary trial endpoint was the change in the number of heavy-drinking days*1 from baseline. A key secondary endpoint was the change in total alcohol consumption*2 from baseline. An important objective was to observe the extent to which consumption of alcohol was moderated in individuals with alcohol dependence.
- 1Heavy-drinking days (HDDs): number of days per month with alcohol consumption greater than 60 grams per day for males and greater than 40 grams per day for females
- 2Total alcohol consumption (TAC): Mean average daily alcohol consumption over a one-month period (grams per day)
In the initial 24-week comparative trial, a statistically significant difference (P <0.0001) was observed between nalmefene and placebo for the primary endpoint, the number of heavy- drinking days from baseline to week 12. A difference was observed for both the 10 mg per day and the 20 mg per day nalmefene groups compared to the placebo group. The effect was maintained through the 24 weeks of treatment.
In the comparative trial, a statistically significant (P <0.0001) decrease was also observed for both nalmefene groups on the key secondary endpoint, total alcohol consumption.
In the long-term extension study, both the number of heavy drinking days and total alcohol consumption decreased through to the end of the 24-week study period.
Side effects from nalmefene included nausea, dizziness and somnolence and these were mild or moderate in intensity. Their frequency or severity did not increase during the long-term extension study. Dependency and withdrawal symptoms from nalmefene were not observed.
More in-depth results on the trial will be presented later this year at a conference in Japan.
Among mental disorders, alcohol dependence not only impairs one's health but also has significant social and economic impacts, reinforcing the urgency to find new ways to help address the issue. Nalmefene is under co-development in Japan by Otsuka and Lundbeck as the first treatment that can be taken when a concern about, or the urge itself arises to consume alcohol. It is an antagonist that binds to and blocks opioid receptors that exist widely in the central nervous system. Under the concept of "going out with sake well", this drug candidate is anticipated as a new, potentially continuous treatment option for the purpose of reducing alcohol consumption and helping patients aim at social reintegration.
About nalmefene
Nalmefene is a selective opioid receptor ligand. Opioid receptors are widely distributed in the central nervous system and govern the intracerebral reward system, emotional control, pain control. Three subtypes (μ or mu; κ or kappa; δ or delta) are known.
Nalmefene is believed to act as an antagonist on μ and δ receptors and to act as a partial agonist on κ receptors, reducing the urge to consume alcohol.
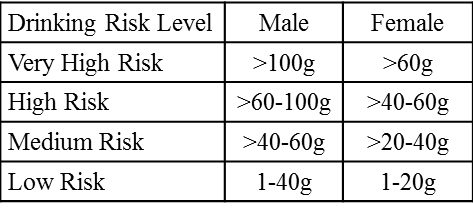
Nalmefene has been marketed in Europe since April 2013 under the brand name Selincro®. The treatment indication in the EU is to help reduce alcohol consumption in adults with alcohol dependence who consume more than 60 g of alcohol per day (for men) or more than 40 g per day (for women), consumption which places the health of these adults at high risk or very high risk as reflected in the table below.