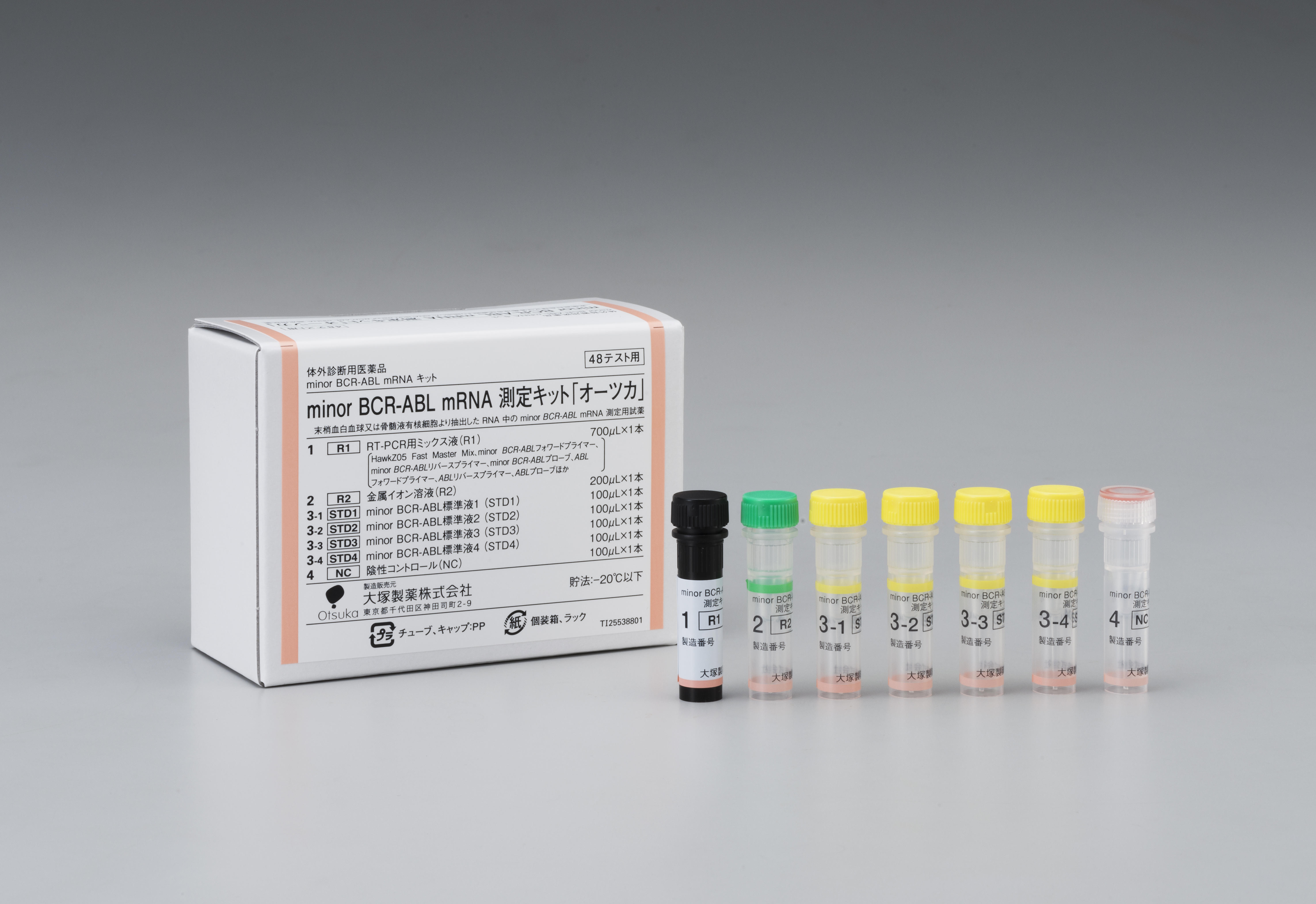
The kit is the first in-vitro, minor BCR-ABL mRNA diagnostic pharmaceutical product in Japan to support diagnosis and to monitor treatment effectiveness for Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Otsuka expects that the kit can support identification of the most appropriate treatment opportunities for patients in Japan with ALL.
In patients with ALL, lymphocytes (a type of white blood cell) become malignant at an early stage of differentiation and proliferate unrestrainedly without maturing into functional lymphocytes. Early diagnosis and treatment initiation are important because symptoms can appear suddenly and the condition progresses rapidly. The number of patients in Japan was reported to be about 5,000 in a survey by the Japanese MHLW in 2017.