Otsuka Pharmaceutical Co., Ltd.
Otsuka Pharmaceutical Confirmed Clinical Efficacy of Rotigotine Transdermal System
for Restless Legs Syndrome in Phase III Clinical Study in Japan
Tokyo, Japan, October 20, 2011 - Otsuka Pharmaceutical Co., Ltd. (Head Office: Chiyoda-ku, Tokyo, Japan; President: Taro Iwamoto) today announced that is has confirmed the efficacy and safety of rotigotine, a dopamine agonist currently under development, for the treatment of idiopathic restless legs syndrome (below, "RLS") in a Phase III clinical trial conducted in Japan. The results of the clinical trial were announced at Worldsleep2011 and the 36th Annual Meeting of the Japanese Society of Sleep Research, jointly held in Kyoto, Japan between October 15 and October 20, 2011.
Rotigotine, dopamine agonist, comes in a compact and thin once-daily transdermal patch. This transdermal patch makes it possible to maintain stable plasma drug levels with continuous 24-hour control of symptoms, including calf itchiness as well as inner leg itchiness, burning and pain. Although RLS symptoms grow worse when lying in bed prior to going to sleep, symptoms also appear during the day when sitting for long periods of time, such as at business meetings. By providing full-day symptom control, rotigotine helps alleviate these symptoms and also improves sleeping disorders and quality of life (QOL). Otsuka Pharmaceutical has promoted the development of rotigotine as a treatment for RLS because of its belief that the drug represents an optimal treatment method for the disorder.
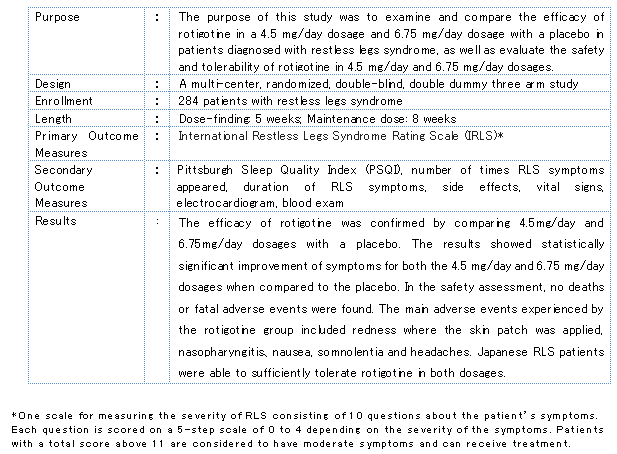
The Phase III clinical trial had an enrollment of 284 RLS patients and compared the efficacy of rotigotine in a 4.5 mg/day dosage and 6.75 mg/day dosage with a placebo, with both the 4.5 mg/day and 6.75 mg/day dosage groups witnessing significant improvement of symptoms over the placebo group. As for safety, with the exception of some minor localized reactions seen in the rotigotine group, such as redness where the skin patch was applied, Japanese RLS patients were able to use the drug safely.
Otsuka Pharmaceutical plans to apply for marketing approval of rotigotine in Japan for the treatment of Parkinson's disease (Phase III clinical study announced in June this year) and RLS before the end of fiscal 2011.
Rotigotine is marketed by the Belgian corporation UCB under the brand name Neupro® in the European Union and other regions and is used to treat Parkinson's disease and RLS.
Otsuka Pharmaceutical acquired the exclusive rights to develop and distribute rotigotine in Japan from UCB in 2002
Based on its corporate philosophy of "Otsuka-people creating new products for better health worldwide," Otsuka Pharmaceutical Co., Ltd. is dedicated to contributing to the health of people around the world.
[Reference Information]
Overview of the Rotigotine Phase III Clinical Trial in Japan
About Restless Legs Syndrome (RLS)
RLS is said to occur in between 2 to 5 percent of the adult population, with most patients over the age of 40, and women in particular having a high prevalence rate. RLS affects the QOL of approximately 2 million patients, yet in most cases RLS is misdiagnosed as a sleeping disorder or other disease because few patients know about it. This also means that many patients today do not receive sufficient treatment for the disorder. Some studies have reported that 1 out of 10 patients diagnosed with a sleeping disorder actually have RLS.
RLS, which primarily manifests itself in the legs (back of the leg, calves and thighs, etc.) as an abnormal sensation, is a chronic disorder that makes it difficult to sit still. The abnormal sensation varies widely by patient, with some complaining of itchiness in their calves or itchiness, burning or pain along the inside of their legs, and symptoms become worse when sitting or lying down, appearing most often before bed. As such, RLS is known to cause sleeping disorders as well. Since this abnormal sensation temporarily disappears when walking or moving the legs, patients become impatient and find it hard to cope with the disorder. Because symptoms manifest when lying down, patients are forced to constantly get up and walk around to alleviate the sensation in their legs, which not only worsens their sleeplessness, but also can lead to mental disorders such as depression. Yet, because RLS is still not widely recognized, it is presumed that many patients are not receiving sufficient medical treatment.
Dopamine agonists have been clearly shown to have the highest efficacy in treating RLS. Dopamine agonists were first developed as a treatment for Parkinson's disease, and are known to alleviate the symptoms of RLS patients in lower dosages compared to Parkinson's disease patients.





