Otsuka Pharmaceutical Co., Ltd.
Otsuka to Co-market New Diagnostic Test for COVID-19, Quick Navi-COVID 19 Ag
-Confirmation of test results in just 15 minutes, and easy-to-read results shown in two contrasting colors-
Otsuka Pharmaceutical Co., Ltd. (Otsuka) and Denka Company, Limited (Denka) will co-market the rapid-diagnostic test kit QuickNavi™- COVID-19 Ag to medical institutions across Japan. The kit, manufactured domestically by Denka, will be co-marketed by Otsuka from September 1 as the latest product in Otsuka's QuickNaviTM series of in vitro diagnostics. It will be launched by Denka prior to Otsuka's roll-out on September 1.
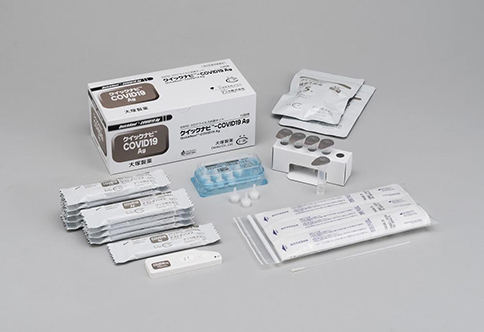

The QuickNavi™- COVID-19 Ag diagnostic kit utilizes a swab to collect nasal secretion samples from the back of the nose and throat (nasopharyngeal swab). Next, the presence or absence of coronavirus antigens in the swab sample is confirmable in 15 minutes through the latex-agglutination method of immunochromatography.
The test outcome is easily discernible in the form of a red test line, which contrasts well with a blue control line. The kit is storable at a temperature between 2 and 30°C and it has a shelf life of 12 months from the date of manufacture, making it easy to handle even in non-specialized medical institutions. Denka is developing a mass production system for 100,000 tests a day to support the production.





