Otsuka Pharmaceutical Co., Ltd.
Phase III Study Results for Rebamipide Ophthalmic Suspension for Dry Eye Announced at ARVO 2011
Tokyo, Japan, May 6, 2011 -- Otsuka Pharmaceutical Co., Ltd. today announced results of a phase III clinical study of its in-development dry eye treatment "rebamipide ophthalmic suspension" for dry eye patients, at ARVO* 2011 (May 1-5, 2011, Fort Lauderdale, Florida, USA).
- * ARVO: Association for Research in Vision and Ophthalmology
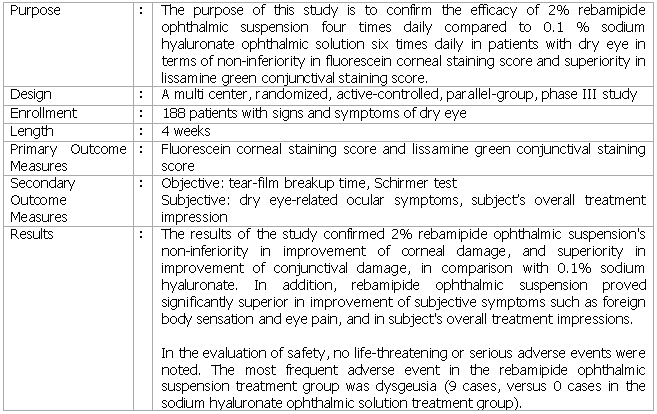
The phase III study was conducted in Japan on 188 patients with signs and symptoms of dry eye, to examine the efficacy and safety of 2% rebamipide ophthalmic suspension in comparison with 0.1% sodium hyaluronate ophthalmic solution. As the results of the study, it was confirmed that in addition to the improvement in corneal-conjunctival damage in patients with dry eye, rebamipide ophthalmic suspension also showed improvements in subjective symptoms such as foreign body sensation and eye pain and in subject's overall treatment impressions.
Based upon the results of this study, in October 2010 Otsuka Pharmaceutical applied for regulatory approval to manufacture and market rebamipide ophthalmic suspension in Japan. In the U.S., a phase II program with co-development partner Acucela Inc. is ongoing.
Based on its corporate philosophy of "Otsuka-people creating new products for better health worldwide," Otsuka Pharmaceutical Co., Ltd. is dedicated to contributing to the health of people around the world.
About the Overview of Study
About Rebamipide Ophthalmic Suspension
By its novel mechanism of action to promote the production of mucin in the ocular surface (both the cornea and the conjunctiva), rebamipide ophthalmic suspension stabilizes the tear film and has demonstrated effectiveness in dry eye treatment. In a clinical trial conducted in Japan, it was confirmed that in addition to the improvement in corneal-conjunctival damage in patients with dry eye, rebamipide ophthalmic suspension also showed improvements in subjective symptoms.
Rebamipide, the active ingredient in rebamipide ophthalmic suspension, was discovered by Otsuka Pharmaceutical and first launched as Mucosta Tablets 100 in 1990 in Japan as an anti-gastric ulcer agent. In 1994, a supplemental indication for the treatment of gastric mucosal lesions (erosion, bleeding, reddening, edema) stemming from gastritis (acute gastritis, acute exacerbation of chronic gastritis) was approved. While exploring the potential of this compound, Otsuka Pharmaceutical focused on the mucin-increasing mechanism of Rebamipide, and rebamipide ophthalmic suspension was developed as a novel dry eye treatment that acts on the decreased amount of mucin in the tear, resulting in dry eye.
Otsuka Pharmaceutical Co., Ltd. has applied for regulatory approval in Japan to rebamipide ophthalmic suspension for treatment of dry eye in October, 2010, and the application is currently under being reviewed.
About dry eye
Dry eye is one of the most common problems treated by ophthalmologists. It is thought that in people with dry eye, the amount of mucin in the tear decreases, resulting in instability in the aqueous layer covering the mucin layer, leading to damages to the corneal and conjunctival epithelia. Additionally, people with dry eye suffer from unpleasant sensory symptoms such as "dryness," "foreign body sensation," and "eye pain," as well as subjective symptoms relating to vision, including "difficulty seeing" and "blurred vision." Such symptoms vary considerably in degree but often interfere with dry eye sufferers' daily lives.





