Otsuka Pharmaceutical Co., Ltd.
Results of two trials show a rapid and sustained response with certolizumab pegol for treatment of rheumatoid arthritis (J-RAPID and HIKARI Studies) released at 2011 meeting of American College of Rheumatology (ACR)
PEGylated*1 anti-TNF-alpha (tumor necrosis factor alpha) certolizumab pegol, which is jointly developed in Japan by Otsuka Pharmaceutical Co., Ltd. (Head Office: Chiyoda-ku, Tokyo, Japan; President: Taro Iwamoto) and UCB (UCB Japan, Head Office: Tokyo, Japan, President and Representative Director: Joel Peterson), was investigated in two Japanese clinical trials for the treatment of rheumatoid arthritis (RA). The results were presented at the American College of Rheumatology Annual Meeting held in Chicago, USA (ACR/ARHP2011*2, November 5th - 9th, 2011). The two trials, conducted as a phase II/III and a phase III, investigated the efficacy and safety of certolizumab pegol.
- *1:Antibody is conjugated with polyethylene glycol (PEG)
- *2:The American College of Rheumatology (ACR)/ The Association of Rheumatology Health Professionals (ARHP) 2011 Annual Scientific Meeting
Results of the two Japanese clinical trials, phase II/III J-RAPID Study*3 and phase III HIKARI Study*4, showed treatment with certolizumab pegol was associated with a rapid and sustained reduction in RA signs and symptoms and inhibition of joint damage progression. Utilizing these two data, UCB plans to file a marketing authorization application for certolizumab pegol in Japan by early 2012.
In the J-RAPID study, certolizumab pegol plus MTX was associated with a rapid and sustained reduction in RA signs and symptoms and inhibition of joint damage progression compared to placebo plus MTX in Japanese RA patients with an inadequate response to MTX*3. In the HIKARI study, of Japanese patients in whom MTX could not be administered, treatment with certolizumab pegol demonstrated a significantly improved clinical symptoms and inhibition of joint damage progression compared to placebo*4. In the HIKARI study analyzed in detail, improvement of clinical symptoms and inhibition joint damage progression was also observed both in patients receiving certolizumab pegol as monotherapy and in those receiving concomitant disease modifying antirheumatic drugs (DMARDs) *4.
- *3:Japanese RA Prevention of Structural Damage; a study in Japanese RA patients for bridging from overseas RAPID study. With concomitant MTX
- *4:Phase III study to assess efficacy, safety and pharmacokinetics of CDP870 (certolizumab pegol) in Rheumatoid Arthritis patients; A clinical trial unique to Japan. Without concomitant MTX
Certolizumab pegol is the world's first PEGylated anti-TNF-alpha (tumor necrosis factor alpha) antibody drug for the treatment of RA. Certolizumab pegol has a high affinity for TNF-alpha, a factor involved in the onset and exacerbation of inflammatory diseases, such as RA, and selectively inhibits the effects of TNF-alpha. Certolizumab pegol has an extended blood half-life due to PEG moiety attached to its Fc-free Fab region*5, and is effective as subcutaneous injection every two weeks or every month in the treatment of RA. In global clinical trials, co-therapy with certolizumab pegol and methotrexate (MTX) rapidly improved signs and symptoms of RA continued to be effective during induction and maintenance therapy. The certolizumab pegol therapy also prevented the progression of joint destruction.
- *5:Antibody is a Y-like shape molecule, comprised of two antigen-recognizing Fab regions and a complement-binding Fc region.
In June 2008, Otsuka Pharmaceutical and UCB Group have signed a contract to jointly develop and market the anti-TNF-alpha certolizumab pegol. Certolizumab pegol has been marketed by UCB Group under the brand name Cimzia® for the treatment of Crohn's disease and rheumatoid arthritis in US, EU and other regions.
Reference information
Outline of phase II/III Japanese RA trial with certolizumab pegol (J-RAPID Study)
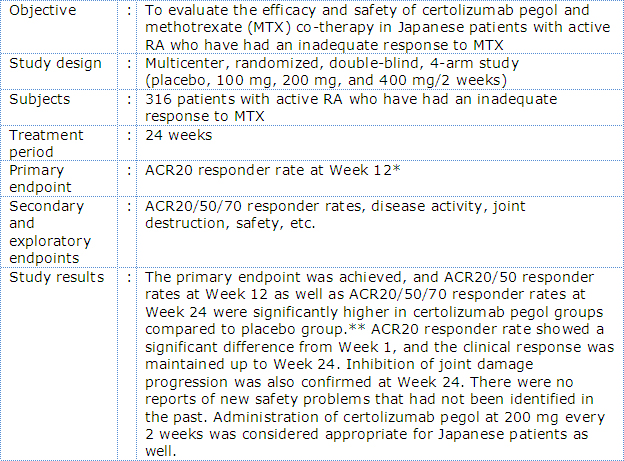
Outline of phase III Japanese RA trial with certolizumab pegol (HIKARI Study)
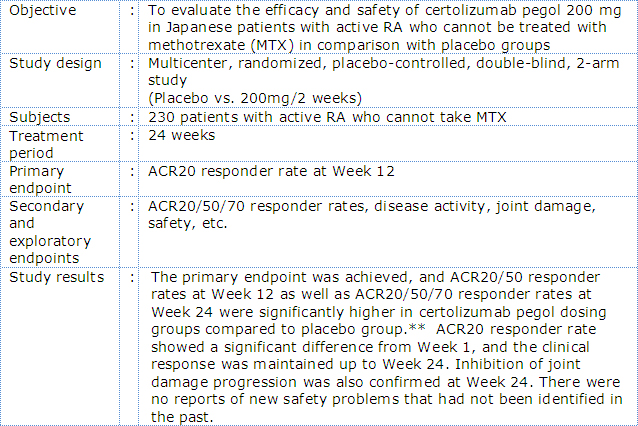
- *:ACR20 response criteria were developed and revised in 1987 under the initiative of American College of Rheumatology (ACR) and are used for the assessment of RA trial results. The ACR core set is comprised of 7 components (tender joint count, swollen joint count, patient's assessment of pain, patient's global assessment of disease activity (PGA), evaluator's global assessment of disease activity (EGA), functional disability, and acute inflammatory reaction), and ACR20 responder is defined as a subject with ≥20% improvement from baseline in both tender and swollen joint counts, and ≥20% improvement from baseline in 3 of the remaining 5 components. ACR50 and ACR70 responders are similarly defined with each corresponding percentage of improvement from baseline.
- **:Significance test was not performed for ACR70 responder rate at Week 12 because there was no ACR70 responder in placebo group.
About Rheumatoid Arthritis (RA)
RA is thought to stem from various underlying causes, though its pathogenesis is yet to be elucidated. It is an inflammatory disease primarily manifests as arthritis, more frequently seen in women with a peak age of onset between 30s and 50s, having a morbidity rate of approximately 0.5% to 1%. RA used to be assumed as a malignant disease to have gradual and progressive degeneration of joints after the disease onset, but it is now known that joint damage rapidly progresses during the early stages of onset and that the average life expectancy is shorter in RA patients according to overseas data. In addition, recent attention is focused on social and economic losses associated with RA, and the emphasis is placed on the importance of disease control with early diagnosis and treatment. Under these circumstances, therapeutic trend has changed in recent years to seek suppression of joint damage progression and complete remission instead of conventional therapeutic goals of pain relief, maintenance and improvement of joint function, and improvement of daily activities.





