CNS area Product story

As long as there are unmet patient needs,
our work continues
The difficulty in pinpointing the causes of diseases of the central nervous system presents a challenge to new drug development.
Despite these obstacles, Otsuka Pharmaceutical discovered and developed ABILIFY; ABILIFY Maintena; and REXULTI, an antipsychotic drug with a unique pharmacological profile.
A challenge born from failure
Diseases of the central nervous system include schizophrenia, depression, bipolar disorder, and Alzheimer's disease. The underlying causes of these diseases have yet to be precisely elucidated, making the research and development of treatments extremely challenging. Cures for these disorders remain elusive, while expectations from patients and their families for new, better drugs remain high.
The creation of ground-breaking medicines often involves many failures - but viewed from a new angle, these can sometimes be seen as spurs to try different research approaches. Such was the case when scientists researching non-drowsy antihistamines found that mice fell asleep during tests of drug candidates. Although not suitable as antihistamines, they speculated that the candidates might have an effect on the central nervous system (CNS). Ultimately research pursued a different path that culminated in the discovery of the antipsychotic ABILIFY. Taking untraveled paths has characterized Otsuka's CNS-related research during the past 40 years.
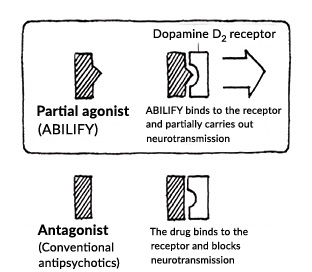
In 2002, we released the anti-psychotic drug "ABILIFY" in the United States, our first original medication. Many antipsychotic drugs that were sold at that time had antagonist effects that inhibited the dopamine D2 receptor. However, ABILIFY works by suppressing dopamine neurotransmission when dopamine secretion is excessive, and activating it when there is deficient dopamine secretion, meaning that it has the world's first dopamine system stabilizer (DSS) mechanism.
CNS diseases and their treatments are often accompanied by adherence issues: patients may lack an understanding of their condition, dislike the side effects of their medication and stop taking it, or forget to take it. This often leads to relapses. For better patient outcomes and improved adherence, desirable antipsychotic medications are effective but also have minimal side effects. Due to its unique characteristics, ABILIFY has gained wide recognition and usage from doctors and patients worldwide, and is now used in approximately 60 countries and regions.
Towards further contributions
Addressing the issue of patient adherence to medication from another direction, Otsuka Pharmaceutical developed the antipsychotic ABILIFY Maintena, a once-monthly injectable suspension. Development of ABILIFY Maintena began before ABILIFY had even gained approval, and production requires sterile production facilities and additional cutting-edge technologies. Lacking these in-house, Otsuka acquired the necessary technology and expertise from scratch. Launched in the U.S. in 2013, and Japan in 2015, the drug is now used in over 50 countries and regions.
In 2023, the bi-monthly formulation (U.S. brand name: ABILIFY ASIMTUFII) was approved by the U.S. FDA for the treatment of schizophrenia and bipolar I disorder.
The research plan for future antipsychotic drugs as successors to ABILIFY began. The new antipsychotic REXULTI (generic name: brexpiprazole) was approved in the U.S. for the adjunctive treatment of major depressive disorder and the indication of schizophrenia in 2015. This drug, is believed to exert a partial agonistic effect on the dopamine receptor D2 and serotonin receptor 5-HT1A and an antagonistic effect on serotonin receptor 5-HT2A (Serotonin-Dopamine Activity Modulator: SDAM). In 2023, it was approved by the U.S. FDA for the first time in the United States for the treatment of agitation in Alzheimer's-type dementia. It is currently used in approximately 60 countries and regions.
Global collaboration in Central Nervous System therapies
In addition to creating new products on our own, we collaborate with other companies to share our strengths and ideas in order to discover, develop and deliver high-value, breakthrough products.
CNS area
In addition to our antipsychotic drugs REXULTI, ABILIFY Maintena and ABILIFY, we hold several marketing rights: Neupro patch, a transdermal dopamine agonist used to treat restless legs syndrome and Parkinson's disease; Selincro, a therapeutic drug treatment to reduce alcohol consumption; and AJOVY, an anti-CGRP monoclonal antibody.





